Cue Health raises $100 million to speed development of rapid, portable COVID-19 diagnostics
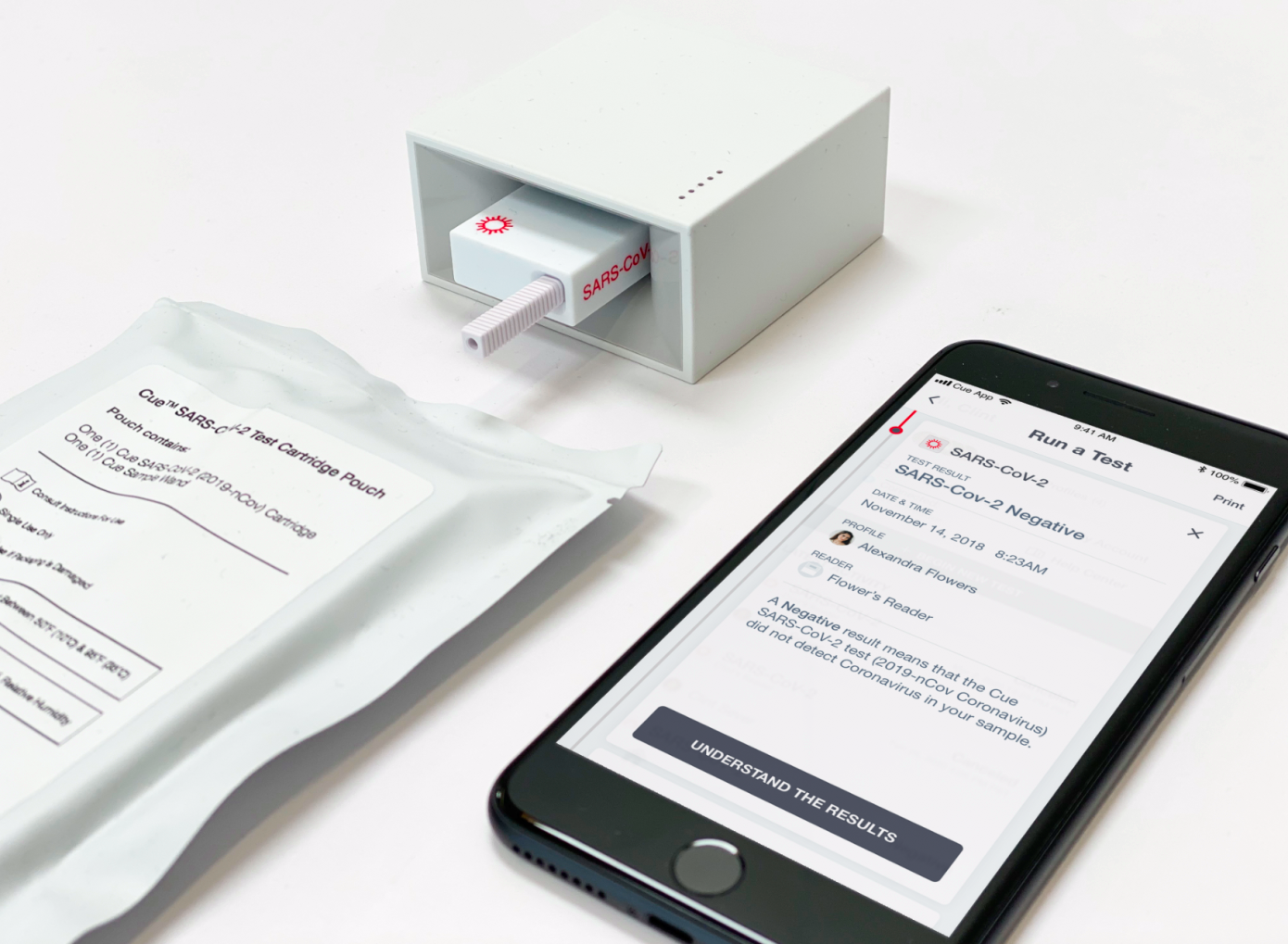
San Diego-based startup Cue Health has closed a $100 million Series C funding round, including participation from Decheng Capital, Foresite Capital, Madrone Capital Partners, Johnson & Johnson Innovation, ACME Capital and others. The funding will be used to help Cue finish up development, validate and scale production of its Cue Health Monitoring System and test cartridges, including test kits designed to diagnose COVID-19, which are currently being reviewed by the U.S. Food and Drug Administration (FDA) for emergency use authorization.
Cue has been developing its connected, portable lab to provide on-site care, including molecular diagnostic tests since its founding a decade ago. The company also secured a $13 million contract in March specifically targeted toward development of a portable point-of-care COVID-19 test. Cue co-founder and CEO Ayub Khattak says that this pandemic has only re-emphasized the need for its product, which is primarily aimed at providing rapid, simple-to-use diagnostics that can be implemented quickly by a wide range of front-line workers in a variety of settings.
Concurrently with its EUA process for the Cue COVID-19 test, the company is also finishing up validation of the results for its influenza A and B tests, which will be used to submit to the agency for final approval of those tests as well. Its tests would provide at-home molecular-based testing, with a connected results platform that can immediately provide diagnostics to certified healthcare providers within 20 minutes, unlocking a new level of remote care.
In total, Cue has $30 million in contracts from the U.S. HHS’ Biomedical Advanced Research and Development Authority (BARDA) across its efforts both for flu testing and for COVID-19 diagnostics.
Original Post: TechCrunch, Darrell Etherington, 6/10/20